Is There a International Review Board for Clinical Research
How do Institutional Review Boards (IRB) and Ethics Committees (EC) impact clinical trials?
- March 16, 2021
- 1:32 pm

Suzanne H.
Sales and Marketing
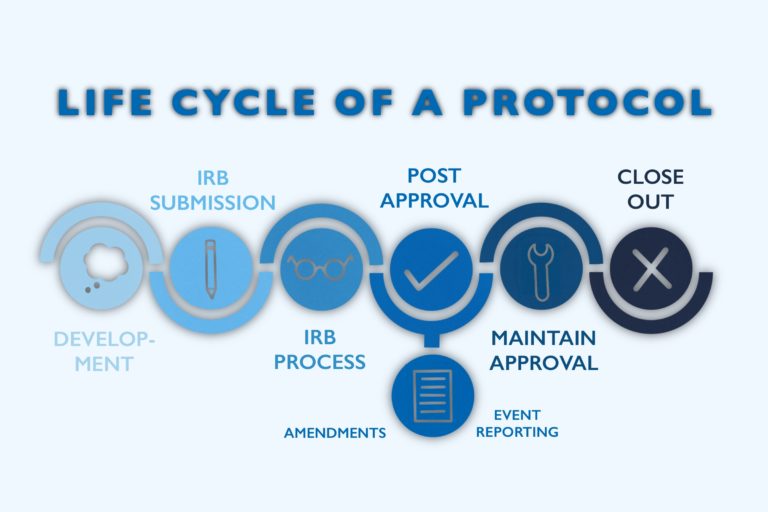
Developing a patient-centric and compliant study is a peak priority for any clinical team. Understanding the importance of Institutional Review Boards (IRB) and Independent Ideals Committees (IEC) and their role is critical to a successful clinical trial. Before a trial tin begin, nevertheless, each aspect of the study must starting time be reviewed and canonical past a designated regulatory body. IRBs and IECs cheque each component of the clinical trial, from protocol evolution to patient reimbursement and travel support. Below, you will larn about several types of these boards and how Clincierge helps sponsors and CROs keep logistics support and reimbursement compliant to avoid whatever written report delays.
IRBs and ECs: Frequently Asked Questions
What is an Independent Ethics Committee (IEC)?
An Independent Ideals Commission comprises various pharmaceutical manufacture stakeholders, including doctors, nurses, social workers, chaplains, and customs members. These members come from diverse backgrounds and are stakeholders in the commitment of healthcare. They join together to discuss the clinical trial's scientific, ethical, and legal ramifications and evaluate the benefit/risk factors involved.
What is the purpose of an Independent Ethics Committee (IEC)?
Ethics Committees ensure that homo subject field inquiry and medical experiments operate according to all national and international laws.
What is an Institutional Review Board (IRB)?
An Institutional Review Board is a formally designated group assigned to monitor and review any medical enquiry involving man subjects. This team has the authority to corroborate or disapprove research and ask for enquiry modifications to lead to approval. IRBs operate nether United states of america Food and Drug Administration (FDA) regulations.
What is the purpose of an Institutional Review Lath (IRB)?
Institutional Review Boards are responsible for protecting and assuring that man research subjects' rights and welfare both before they brainstorm their participation (with advisable protocols in identify) and throughout the clinical trial (through periodic written report reviews).
What is the divergence between an IRB and IEC?
Clinical trials conducted in the European Spousal relationship are held accountable by Independent Ethics Committees (IECs). In the U.s.a., Institutional Review Boards (IRBs) have oversight and must abide by the United States Food and Drug Assistants (FDA) regulations. Countries other than those in the European Union and the United States accept private committees and regulations.
In that location are two types of IRBs and I ECs, local and key. Local comm ittees support individual inquiry institutions and are responsible for reviewing only their clinical trials. Cen tral boards oversee the review of clin ical studies for many different organizations.
More specific guidelines on IRB and IEC responsibilities, composition, functions, and operations are listed in this addendum .
How are IRBs and IECs formed?
Per the FDA, each IRB/ I EC should include 5 or more than members and come from various backgrounds . I of these representatives should come from a non-scientific groundwork and is referred to as a lay member. Another should be an independent member who is not affiliated with the clinical site or medical institution. All members should be competent i ndividuals who are able to understand all aspects of the clinical trial procedure.
The Food and Drug Administration ( FDA ) has compiled a list of oft asked question s and their corresponding answers to provide clarity on United States regulatory procedures . This document explains how IRBs are created , outlines their respons ibilities, and g ives detail on various questions one may encounter during the blessing procedure.
A similar directive has been provided for countries in the European Spousal relationship. Once again , the arduous clinical trial process is broken down into more manage able data, but the reader will continue to realize the numerous hurdles to be overcome.
Why are IECs and IRBs so critical?
Independent Ideals Committees and Institutional Review Boards are necessary to monitor all aspects of clinical trials, ensuring that human trial participants' rubber is their top priority. They review each study through neutral eyes, evaluating the risks and benefits throughout every clinical trial process step.
How long does it take to receive IRB/IEC approvals?
As explained earlier, each aspect of the clinical trial, including patient payment guidelines, must exist approved past the IRB before whatever patient recruitment and enrollment are permitted to brainstorm. Mostly, an IRB submission takes one calendar month to receive approval, with expedited/exempt approvals returned in virtually two weeks. If at that place are any details of the study that do non align with IRB standards, the IRB refuses approval and suggests amendments to receive approval. Just one minor change could issue in weeks or months of delays for a clinical trial, leading to loss of money for the sponsor and loss of much-needed treatment for trial participants.
Each country across the world has its regulations for stipend and per diem payments, patient reimbursements, and tax reporting of income related to clinical trial participation. When conducting a trial at sites in various countries, understanding these unique requirements is hugely fourth dimension-consuming and overwhelming. Often, countries will brand changes to their guidelines, causing the need to rapidly pivot to adapt to these updates and adhere to the new rules.
As your full-service provider of logistical support, Clincierge has the expertise needed to aid appraise the feasibility of supporting patients in specific regions worldwide. We streamline the process by providing required documents in multiple languages as well equally manage whatsoever document revisions. Our squad keeps abreast of current regulations in each country and provides recommendations to ensure compliance and adherence to specific guidelines.
Clincierge has the expertise needed to help you navigate the complexities involved with IRBs and IECs across the globe. These services will ease the burdens associated with your clinical trial regulatory processes. We handle:
- Information Compilation and Document Submissions
- IRB/EC Submissions
- Regulatory Reports
- Study Subpoena Documentation
- Written report Termination Documentation
Remove barriers to clinical trial participation and improve your trial's performance
reynoldsfalit1980.blogspot.com
Source: https://www.clincierge.com/irb-ec-critical-impact-on-clinical-trials/
0 Response to "Is There a International Review Board for Clinical Research"
Post a Comment